A team of physicists from the University of Amsterdam has made significant advancements in the measurement of strontium atoms, achieving unprecedented precision that could enhance modern atomic clocks and quantum computers. By leveraging the properties of neighboring rubidium atoms, the researchers published their findings in the journal Physical Review Letters on November 4, 2025.
Strontium, while not widely known outside scientific circles, is one of six alkaline earth metals and possesses unique properties that make it valuable in various technologies. Among its isotopes, 87 Sr stands out due to its odd number of protons and neutrons, making it a fermion, unlike its bosonic counterparts. This fermionic nature allows for a nonzero nuclear spin, which is crucial for applications in atomic clocks that require precise frequency measurements of emitted or absorbed light.
Applications in Atomic Clocks and Quantum Computing
The 87 Sr isotope is particularly promising for next-generation atomic clocks, known as optical clocks. These clocks rely on the accurate determination of light frequencies emitted during atomic state transitions. For 87 Sr, the optimal frequency occurs at a wavelength of 698 nanometers, corresponding to red light. However, the bosonic isotopes face limitations due to their zero spin, which hinders the necessary emissions. The nuclear spin of 87 Sr allows for a slight modification of these spin rules, enabling effective transitions and contributing to the stability of the clock’s frequency.
The research also delves into the Zeeman effect, discovered by Dutch Nobel laureate Pieter Zeeman in 1896. This phenomenon describes how magnetic fields can cause energy level splitting within atoms, leading to various light frequencies. For the 87 Sr nucleus, the Zeeman effect results in ten distinct energy levels when subjected to a magnetic field, providing foundational elements for quantum computing. While classical computers utilize bits representing binary states, quantum computers exploit qubits, which can exist in multiple states simultaneously, enhancing computational capabilities.
Precision Measurement and Future Implications
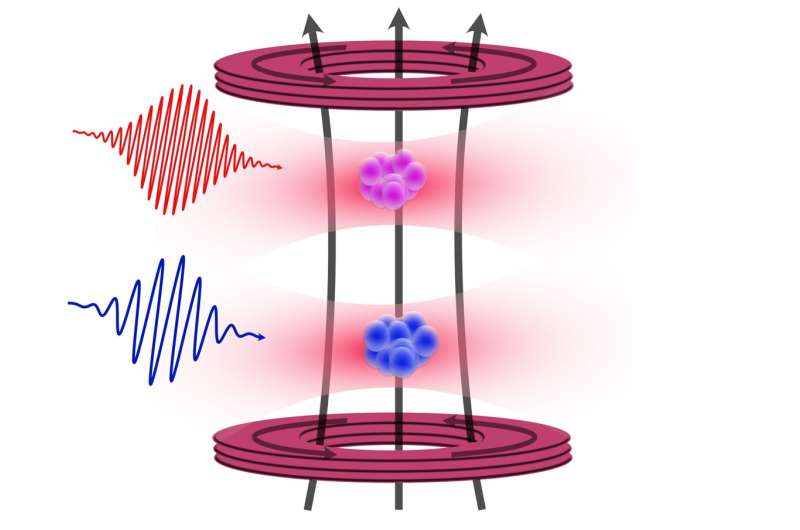
A critical aspect of this research involved determining the strength of the nuclear magnet of 87 Sr—a value expressed as the g-factor. The team, led by first author Premjith Thekkeppatt, improved the previously established g-factor by a remarkable hundredfold. This achievement stemmed from their experiments involving optical trapping of 87 Sr atoms alongside rubidium, allowing for precise calibration of the magnetic field.
Thekkeppatt explained, “The work grew out of our efforts to merge strontium atoms with another element, rubidium, to create rubidium-strontium molecules. This turned out to be extremely challenging, prompting us to investigate what we could achieve by having both species in one another’s vicinity, while avoiding overlap.” Although the initial aim was to create new molecules, the proximity of the two atoms allowed for enhanced measurement techniques, significantly improving the determination of the g-factor.
The new precision established by the Amsterdam physicists not only bolsters the potential of strontium-based applications in atomic clocks and quantum computing but also sets a new benchmark for atomic structure calculations. Thekkeppatt noted that their results could inspire further investigations into other atomic species and states, paving the way for advancements across various scientific domains.
In conclusion, the innovative methods employed by the University of Amsterdam’s research team highlight the intricate relationships between atomic properties and their applications. The findings will likely influence the development of more accurate atomic clocks and the evolution of quantum computing technologies in the years to come.